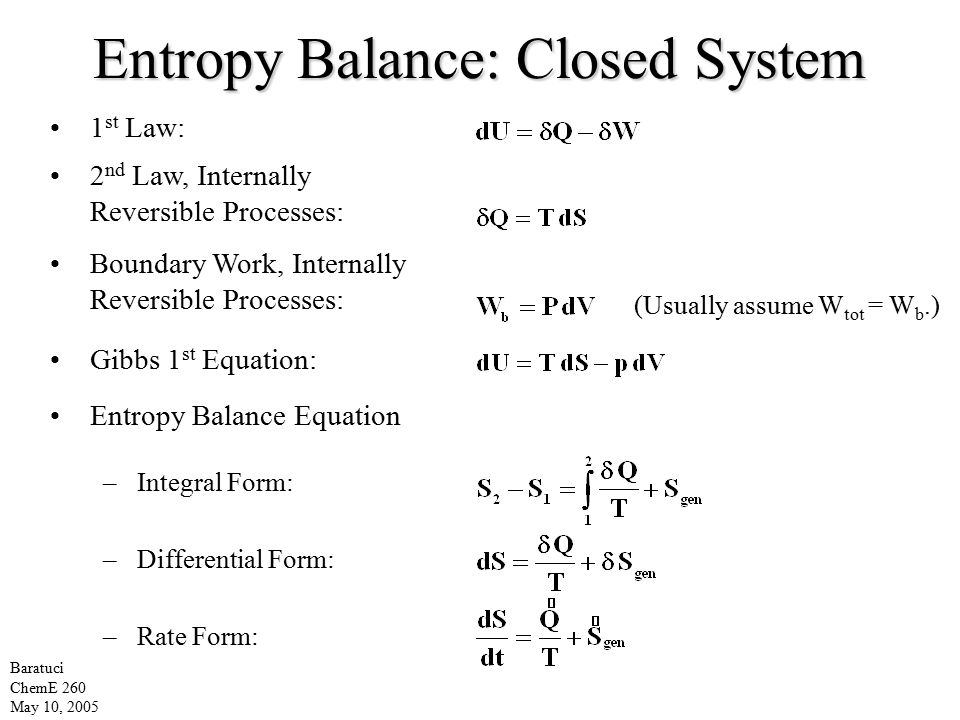
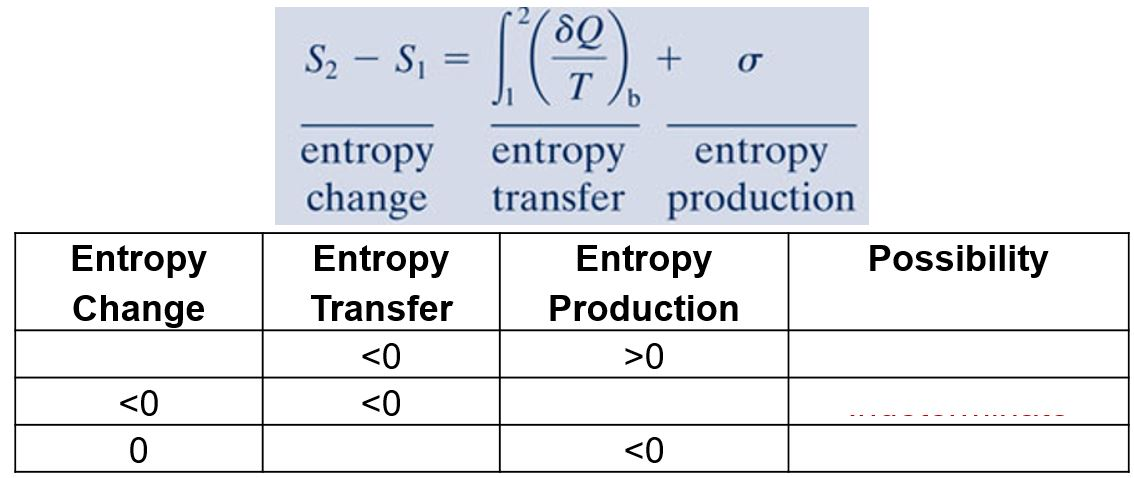
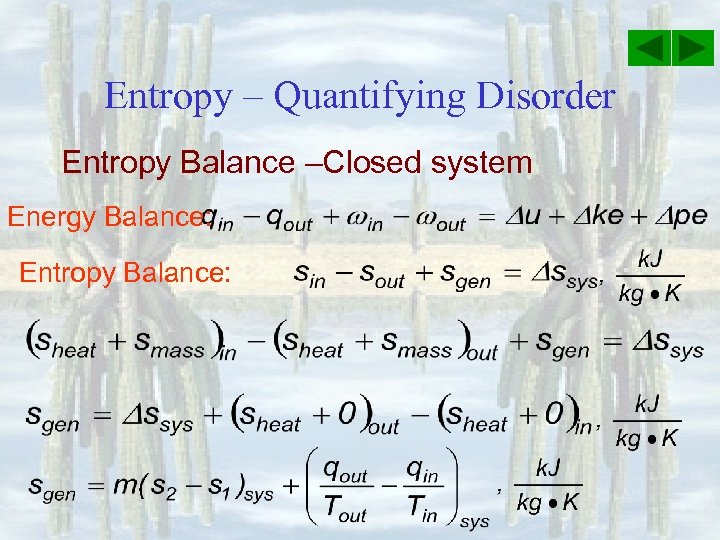
Entropy Balance Closed System
Entropy balance closed system. The entropy balance is reduced to. Entropy Balances on Closed System. Draw a diagram to represent the system showing control massvolume of interest.
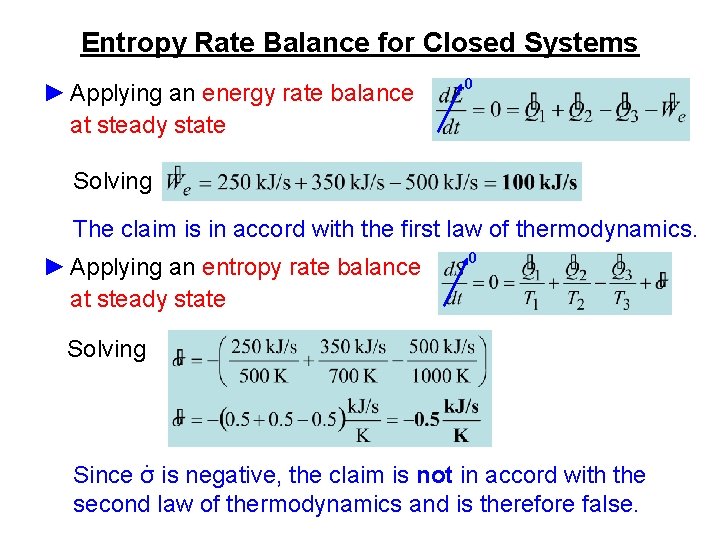
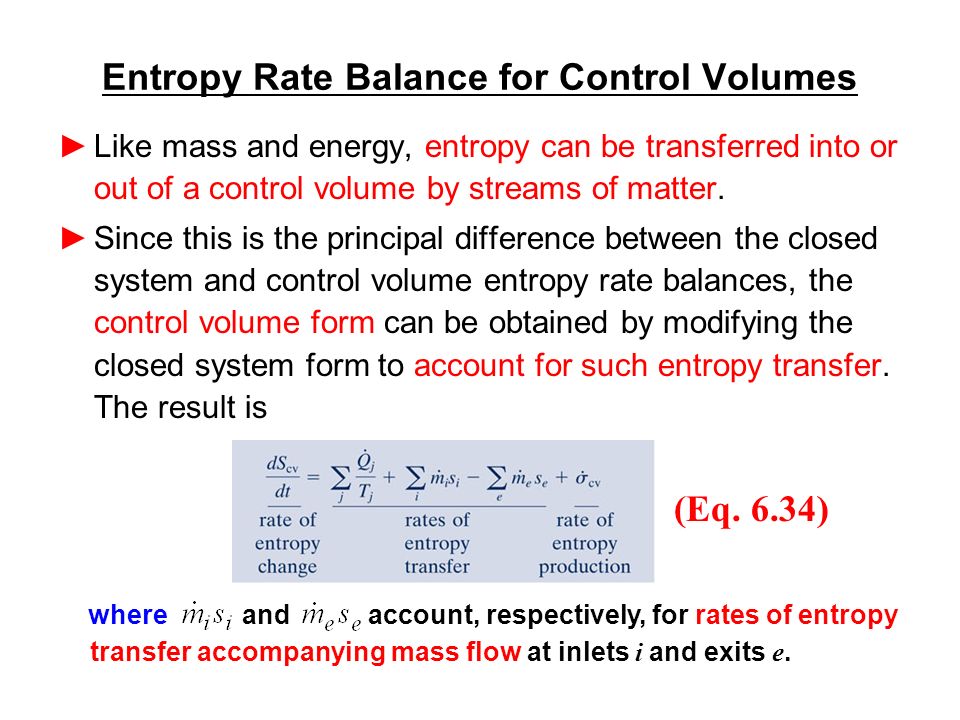
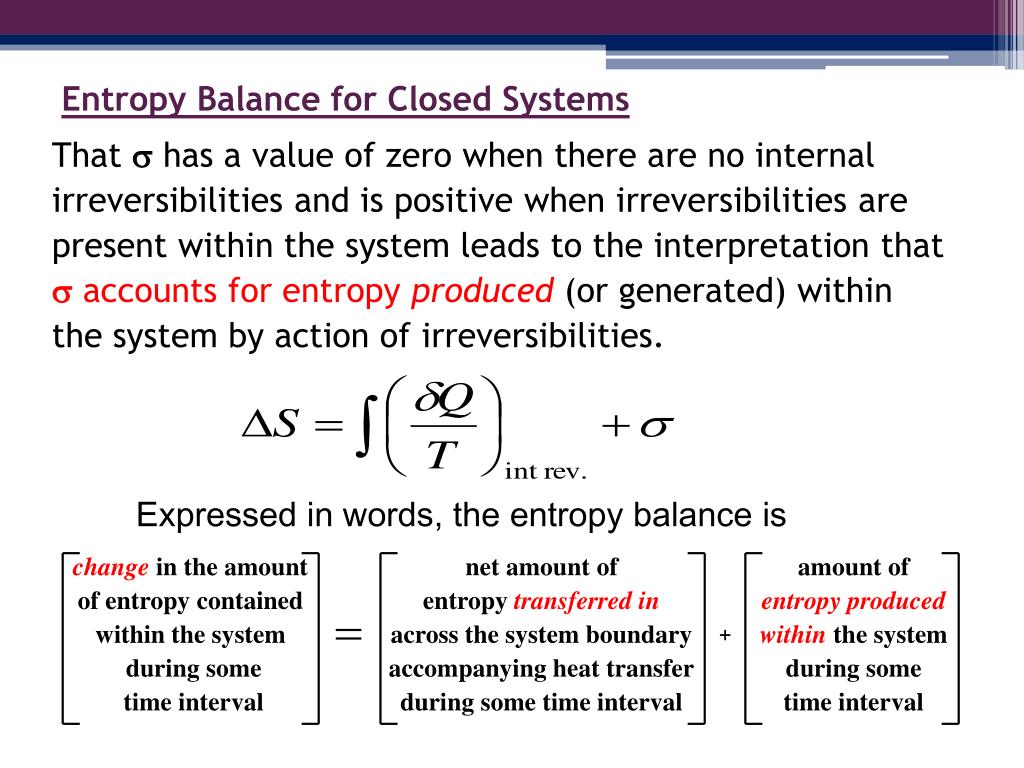
The entropy of a substance can be measured although in an indirect way. If a process occurs in a closed system the entropy of the system increases for irreversible processes and remains constant for reversible process. Using energy and entropy rate balances evaluate the thermodynamic performance of the system.
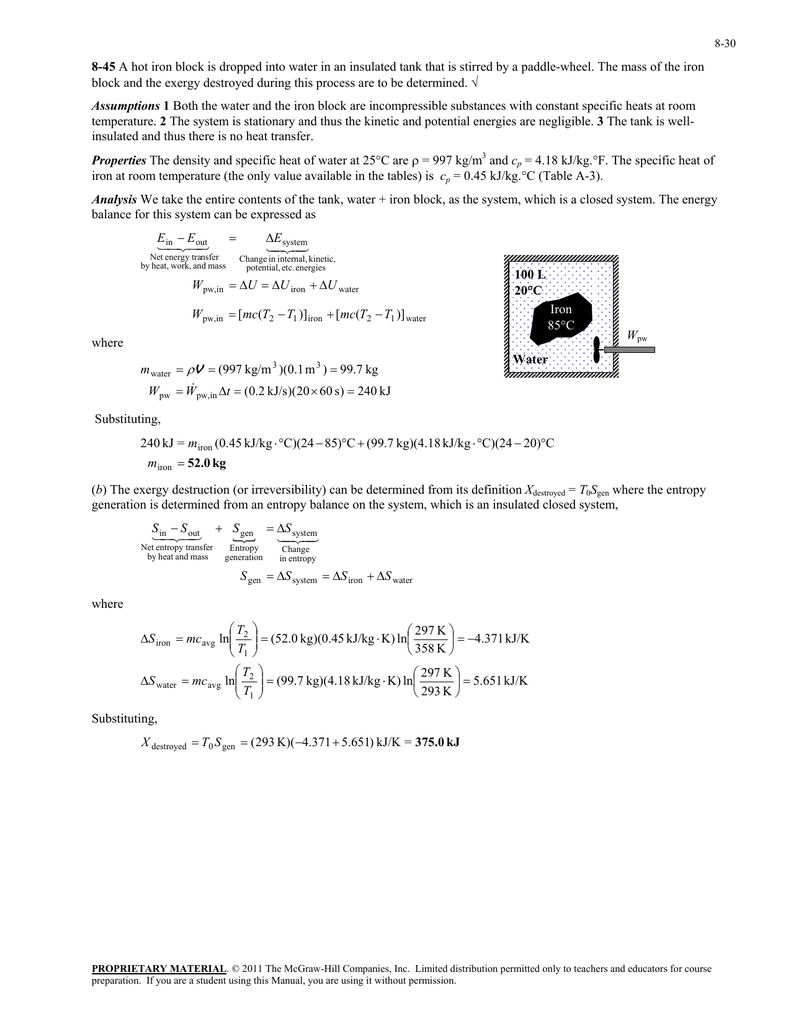
From the entropy balance 002 BtulboR The entropy transfer term must be negative since the change in entropy is negative. Entropy Balance for a Closed System Example 1 Water initially as a saturated from MEMS 0051 at University of Pittsburgh-Pittsburgh Campus. Kinetic and potential energy effects are negligible.
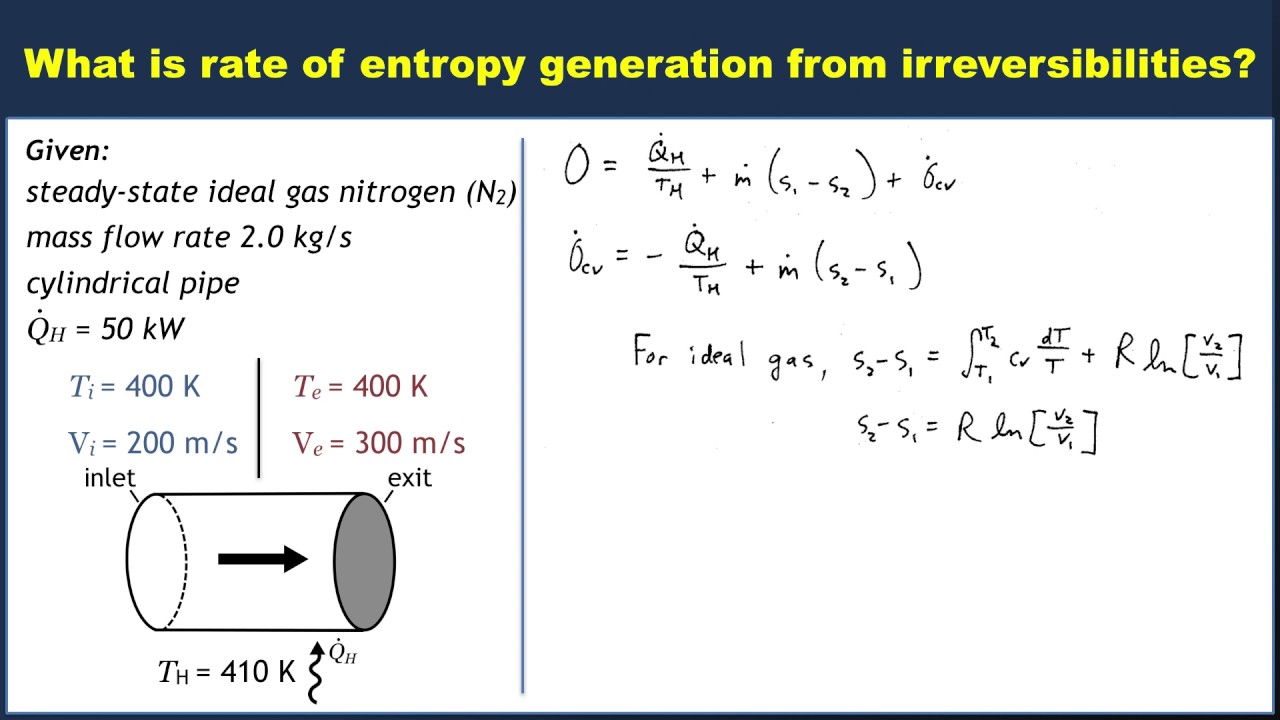
10 o σ 0 T s 1 2 540 oR 700 R. 6102Steam at 550 lbfin2 7008F enters an insulated turbine operating at steady state with a mass flow rate of 1 lbs. Write out what you are required to solve for this is so you dont forget to answer everything the question is asking for Find.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Sgencv - the rate of entropy production within the turbine per kg of steam. At state 1 and state 2 the total energy in a closed system is The exergy of the mass in the closed system at state 1 and state 2 are.
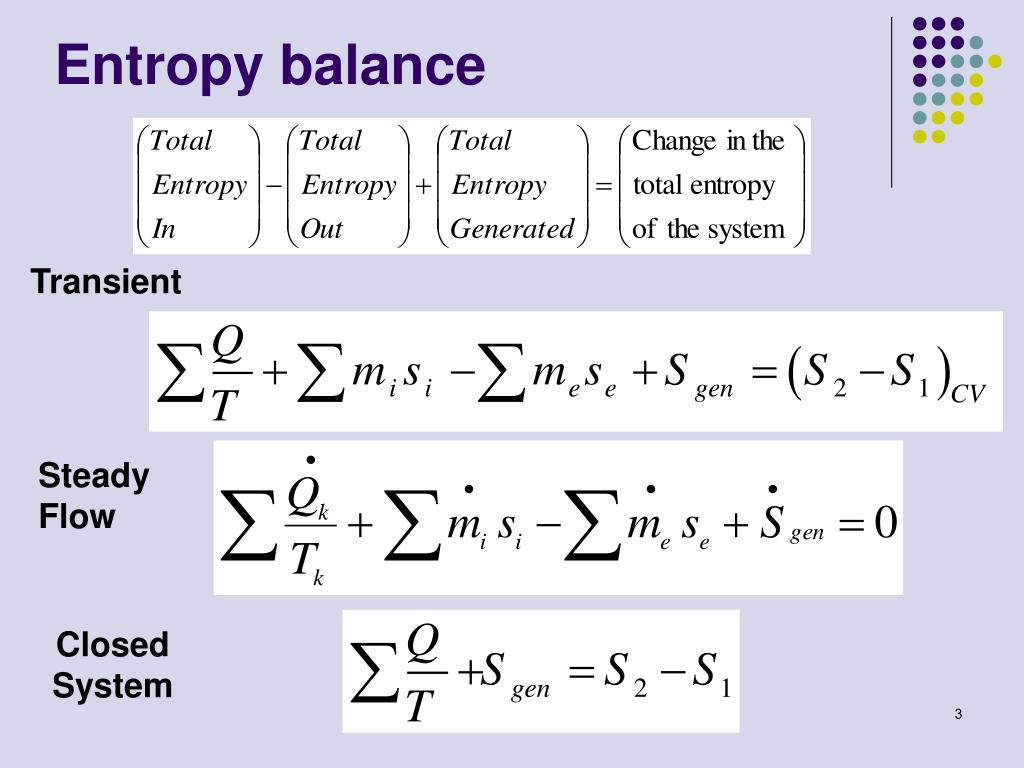
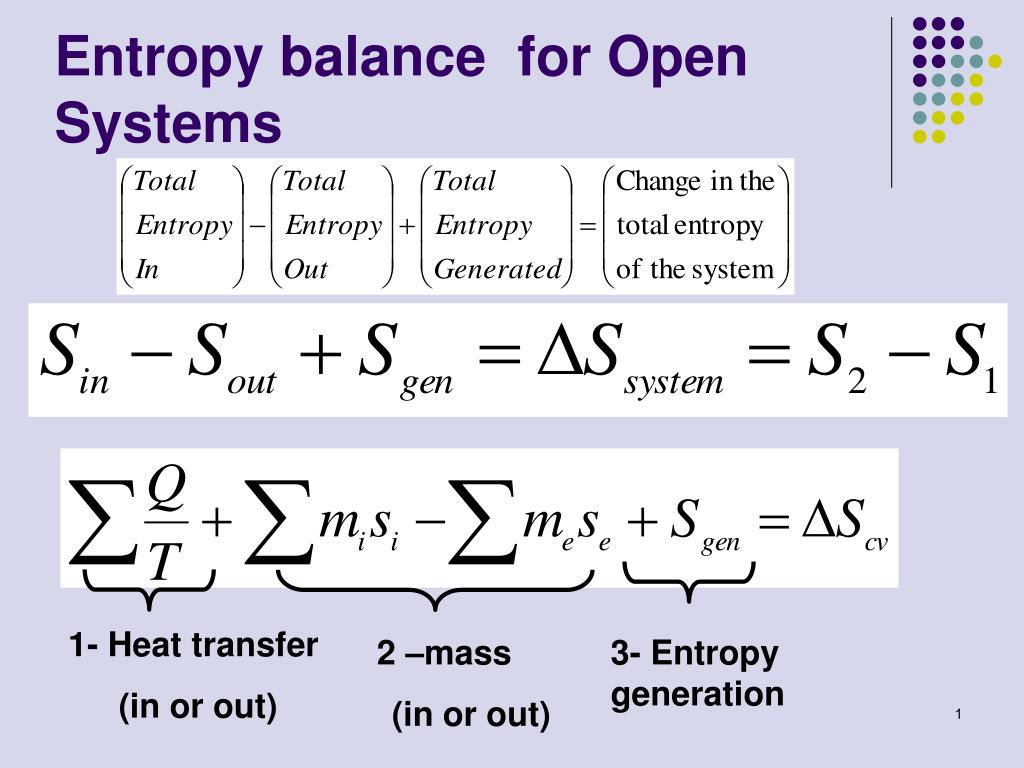
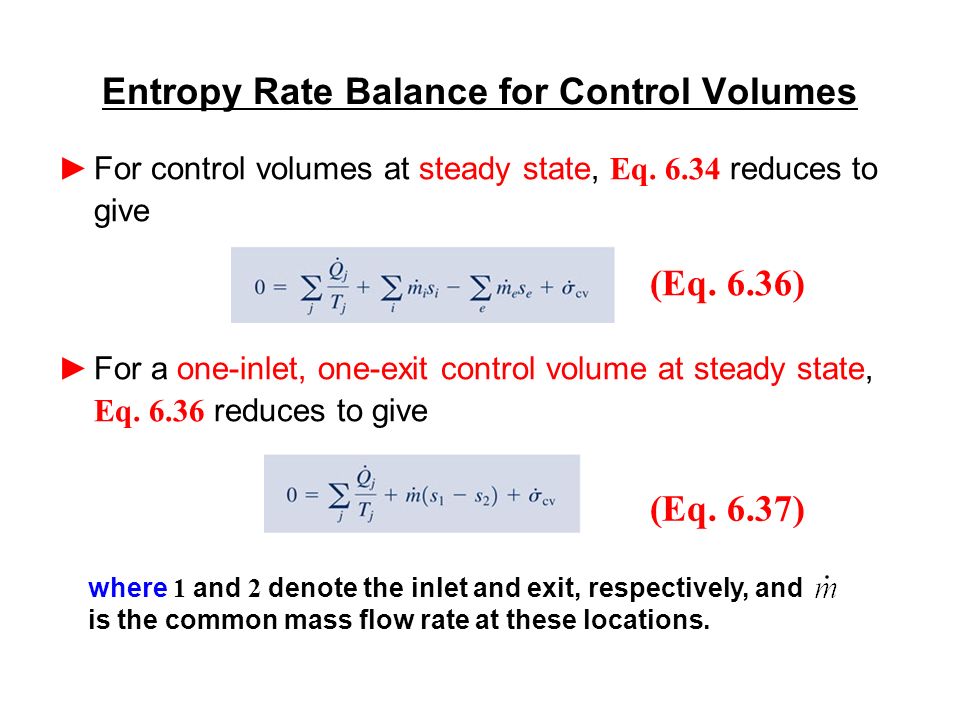
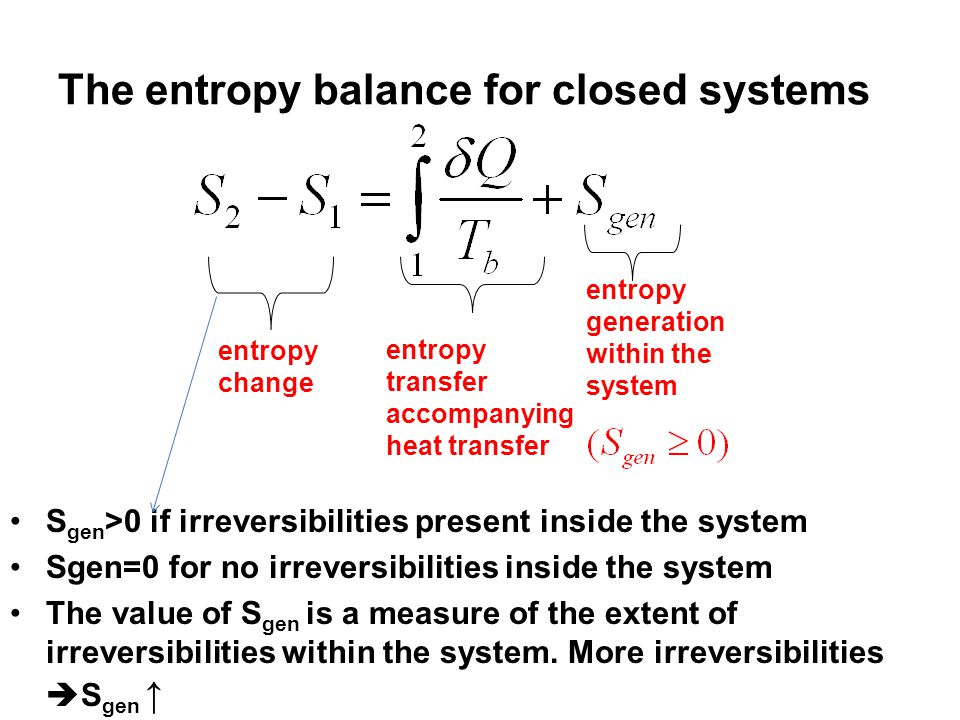
Second Law of Thermodynamics. The entropy change of a closed system is due to the entropy transfer accompanying heat. Let us determine an expression for the entropy production from the entropy balance of a steady system.
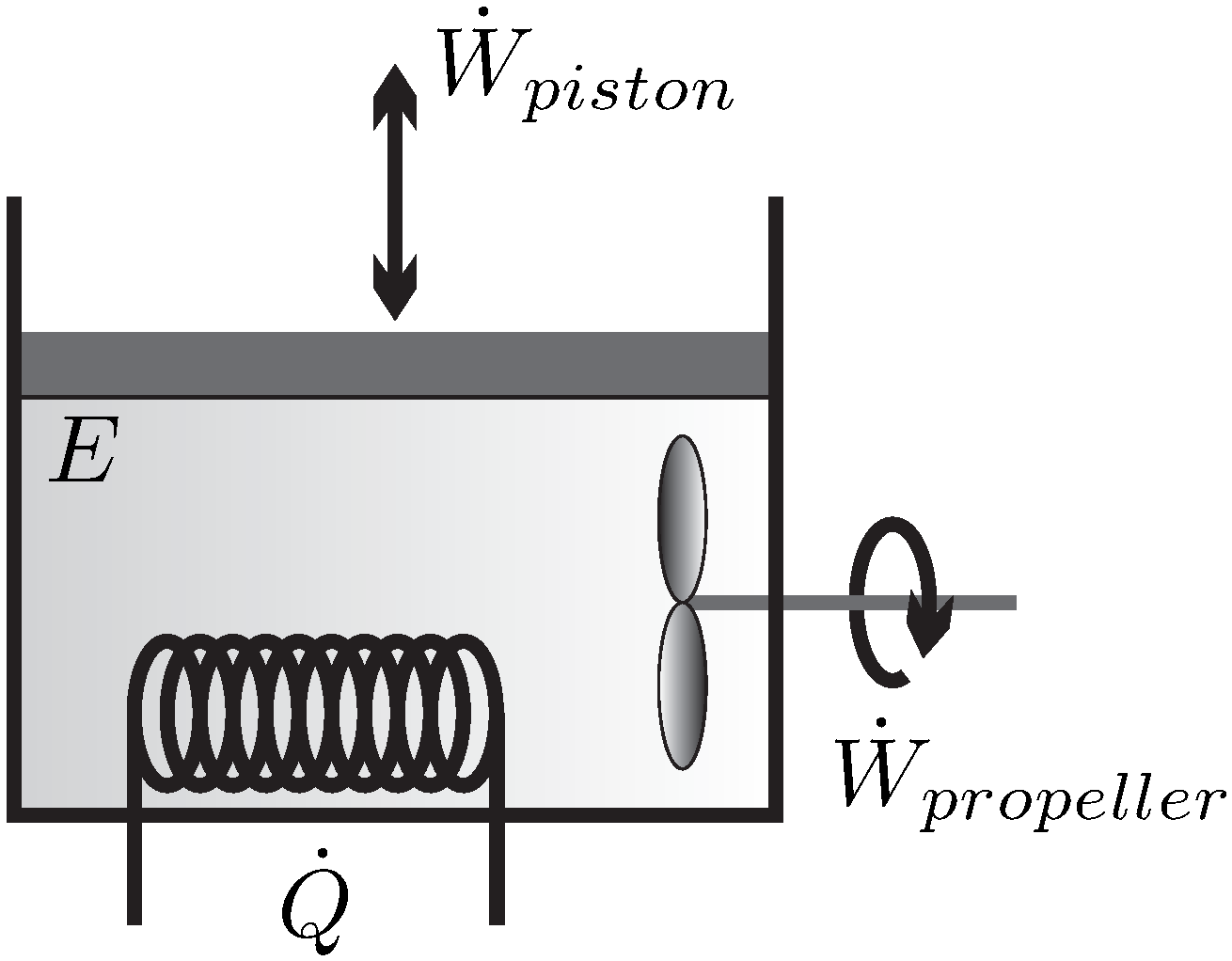
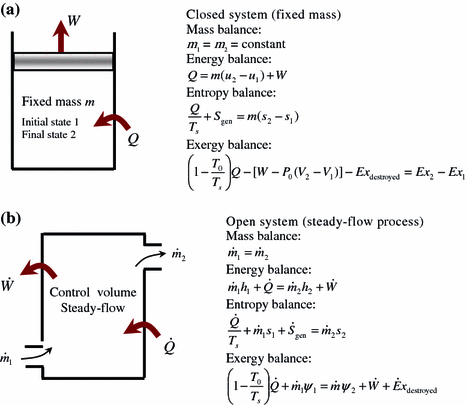
A Closed System. Not a closed system.
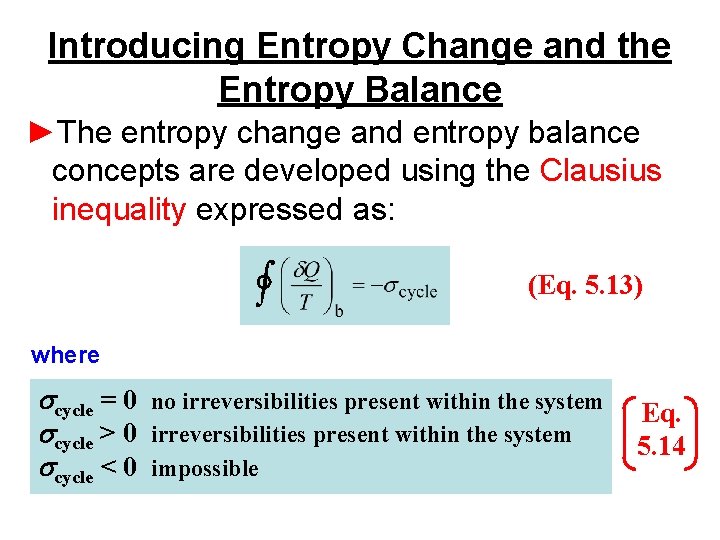
ΔS total 0 If a process occurs in a closed system the entropy of the system increases for irreversible processes and remains constant for reversible processes.
From the entropy balance of an overall system composed of reservoirs and reactor the intensity of the entropy production σs follows in terms of the reservoir parameters and system fluxes. I have explained here in short about what has been given in my textbook for entropy. From the entropy balance 002 BtulboR The entropy transfer term must be negative since the change in entropy is negative. KJ kg m Wcv 540 Step 2. If the closed system undergoes an adiabatic process then no heat transfer occurs at the boundary. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Not a closed system. Second Law of Thermodynamics. Write out what you are required to solve for this is so you dont forget to answer everything the question is asking for Find.
Entropy Balances on Closed System. Entropy production balance of entropy entropy inequality balance equations - closed systems 23 dissipation inequality dissipaltion inequality thermodynamic restriction eg fouriers law. ΔS total 0 If a process occurs in a closed system the entropy of the system increases for irreversible processes and remains constant for reversible processes. Second Law of Thermodynamics. If entropy generated increases the engines efficiency decreases Closed system with steady-state flow Simple Heat Engine Heat Engine ሶ 1 ሶ2 2 ሶ numberofmolesmol internalenergyJ absolutepressurePa absolutetemperatureK shaftworkdoneonsystemJപ𝜃propertyθpermole workdoneonthesystemJ. A closed system contains internal kinetic and potential energies. Balance equations - closed systems 22 balance of entropy.


















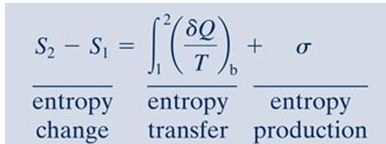



Post a Comment for "Entropy Balance Closed System"